Bitterness is not so much a topic nowadays as it is acidity and fruity flavours. However, bitterness is an essential pillar when it comes to the taste of the coffee. It is hard to achieve a roast of coffee which is not bitter at all. Moreover, I believe it is also not desired to have a non-bitter coffee.
Flavour vs Taste: What’s the difference?
When one speaks about flavour, only the olfactory volatile compounds are meant by that. The flavour is only what we can smell with the receptors in our nose. We can smell it directly through our nostrils or when we have already taken a sip of coffee and the whole spectrum of coffee flavours activate the receptors in our nose via the connection between the mouth and nose. This sensation is called retro-nasal olfaction.
When one talks about taste, one refers simply to the sensation taking place on our tongue. Specifically, there are sour, sweet, salty, umami, and bitterness. Everything else, commonly cited as “strawberry taste” is perceived with our nose and is not a taste but a smell.

The tastes and flavours of coffee are based on the coffee cherry and its chemical composition. The alluring aroma comes to shine when the raw bean is processed in the right way either being naturally or wet-processed or being macerated or really fermented and, of course, the roasting.
Myth-Bust: There is this myth going on, that coffee has over 1000 thousand flavour compounds. This is a myth. There are just 23 key odorants in coffee which make its alluring flavour. Does not sound as much anymore? To set the numbers in a frame: wine has only 15 of these key odorants (= flavor compound). However, the taste of coffee is not as well understood so far. There are thousands, maybe tens of thousands, of taste active compounds all adding up to the beautiful complexity and the fragile balance of sour, bitter, and umami taste in coffee.
Chemical reactions during the roasting
During the roasting, coffee undergoes various reaction pathways. It all starts with fundamental physical changes including evaporation of water and volatile compounds such as low molecular organic acids. However, those evaporating compounds build up pressure on the inside of the coffee bean’s cells. The coffee bean acts as little pressure reactor upon roasting. Basically, the cell wall is fighting against the volume expansion. At one point during the roasting, mainly the first crack, the internal pressure of the bean becomes too high and the cells burst with a noticeable little crack.

During that time, a myriad of chemical reactions hardly understood even until nowadays, take place. Those reactions are named Maillard Reactions and are taken into account for the formation of most of the flavour in roasted coffee. Amino acids, the molecules proteins are made of, react with carbohydrates. Due to the high heat during the roasting, water splits off, and several other rearrangement reactions take place, forming the highly alluring flavour profile of that complex, brown coffee bean.
Building coffee-like bitterness during roasting
However, around 180 °C, uncountable reactions are starting to occur building bitter tastants in coffee. At this point, we come back to the green coffee’s composition. The bitter taste of coffee is hardly dependent on the chlorogenic acid content besides the initial water content of the green coffee bean and its size. Chlorogenic acids are a group of polyphenols and are sour tastants themselves. They occur up to 13 % in green coffee, hardly dependent on the variety, the terroir, and the processing.
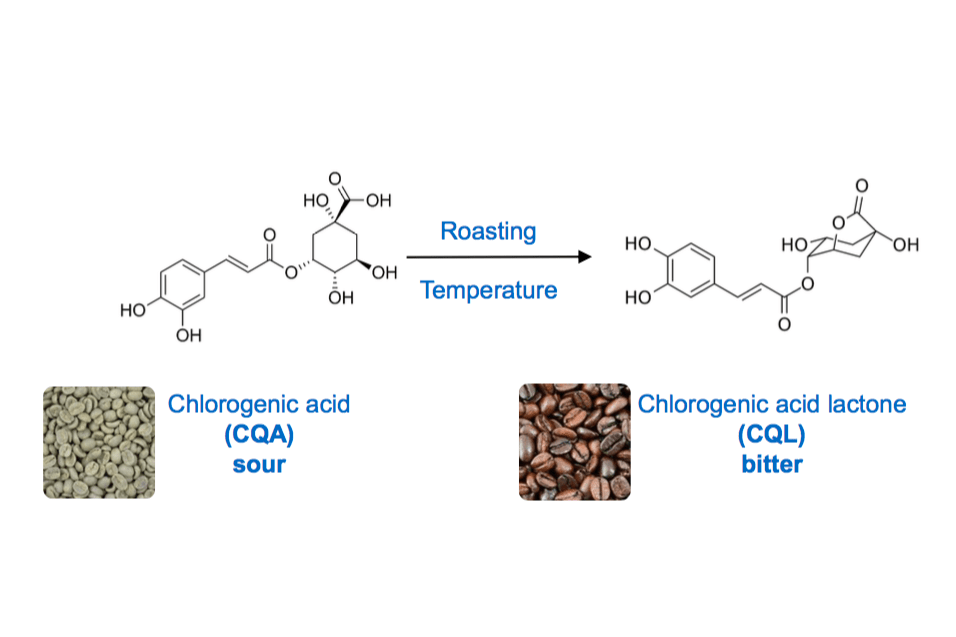
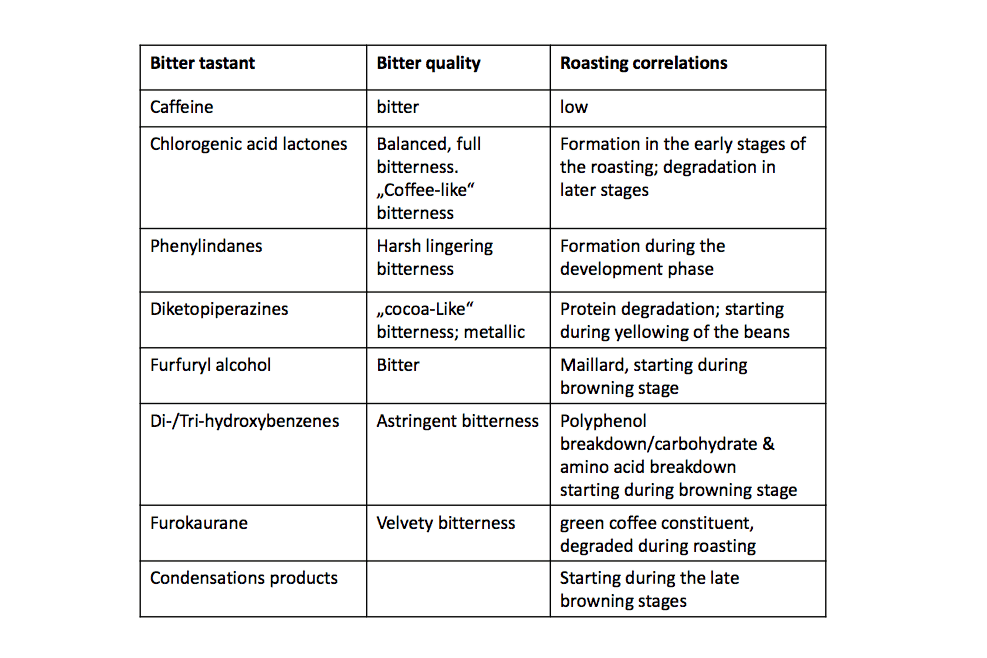
Upon roasting and especially in mid to mid-late stages of the roasting, those chlorogenic acids undergo a reaction called intra-molecular lactonization and generate chlorogenic acid lactones. These lactones, in opposite to the chlorogenic acids themselves, are velvety and pleasantly bitter tastants in coffee. Most of the panellists during my research described lactones as “coffee-like” bitterness and attributed those lactones to a desirable bitter contribution.
Harsh bitterness: It’s the fault of phenylindanes
However, the fragility of the coffee roasting comes into play. Coffee roasting is a skilful process. It demands a lot of experience, science, and a lot of knowledge. So those desirable lactones in coffee, as well as the remaining chlorogenic acids, can undergo further degradation reaction. Once the bean is roasted further, mainly starting with bean temperatures above 210 – 220°C (every bean is unique), the chlorogenic acids and the lactones are further degraded. Both are forming the so-called phenylindanes. These group of taste active compounds were found to be highly potent bitter tastants, contributing in no small amount of the undesirable, harsh, bitter taste in coffee. Sometimes, the phenylindanes are also described as metallic, long-lasting bitter compounds in coffee.
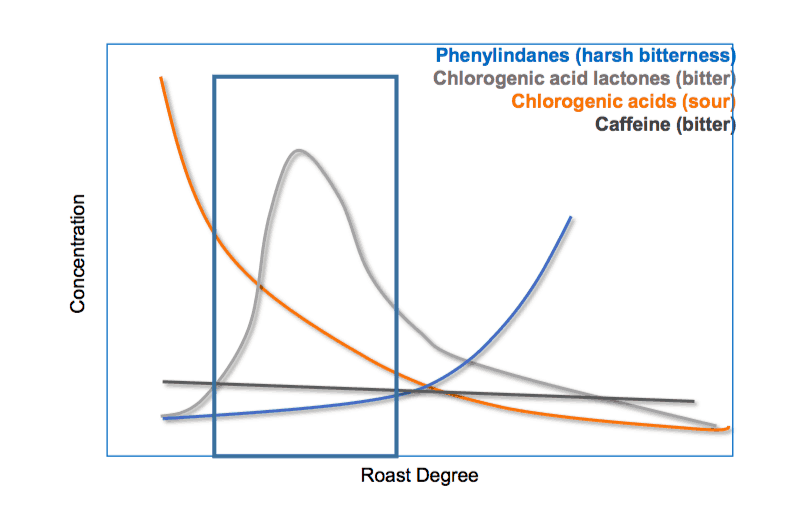
The formation of lactones and phenylindanes underlie two factors: temperature and time. If one is trying to shut the time short, they will always have to increase the temperature. However, a higher temperature will always result in a faster formation of both lactones and phenylindanes. However, if the temperature is decreased, the roasting time will be longer. This results in a slower generation of those bitter tastants. Nevertheless, if one overdoes the time, not only the flavour of the coffee will fade, they will also form more of the undesired phenylindanes.
Hot-air roasting vs drum roasting
One might wonder whether hot-air roasting (convectional) or drum roasting results in a more balanced coffee? Honestly speaking, and this might not sound in line with current “artisanal drum roasting” movement, I would rather pick a hot air roaster than a drum roaster. The main reason for that lies within the heat transfer to the bean.

Convection is more effective in heating the bean through and through than a drum roaster. A drum roaster is based on heat transfer using a mix of both conduction and convection. Conduction is heat transfer by touching a surface whereas convection is heat transfer via air. Speaking of the drum roaster, it could result in a not fully roasted coffee bean. So, the surface of the bean could be already dark and oily and the inside is still underdeveloped. When it comes to flavour, an underdeveloped bean could lead to green and grassy notes mixed with an unpleasant bitter taste of the burnt bean surface.
In the end, it all comes down to the roaster. Nevertheless, a skilful roaster might make more reproducible results on a convectional roaster. This might not sound as alluring and emotional as a drum roaster. I understand that a drum roaster is how the customers want to perceive an artisanal roaster when they consider buying their coffee outside of a supermarket. From a science point of view, I would pick a convectional hot air roaster in the long run.
How bitter is caffeine?
So how about caffeine, one might ask? Caffeine is also a bitter tastant besides keeping us awake and stimulating our central nervous system. Caffeine makes up around 10 – 20 % of the coffee’s bitter taste. It is often cited that caffeine undergoes thermal degradation during the roasting which is not entirely true. Caffeine contents remain mostly the same during roasting (~ 5 – 10 % loss). So yes, caffeine contributes to the overall bitter taste. When it comes to roasting, it does not play a huge role in which kind of setup one uses.
So, what can we draw as a conclusion? Roasting coffee remains art and science. There is no easy answer to the perfect roast. Any roast is highly dependent on the green coffee, its constitution, the processing, the roasters architecture, and the brewing method. The key to a “perfect coffee” is the perfect balance of taste and flavour. In my opinion, the perfect balance lies within mild acidity and mild bitterness with an intense and diverse flavour dependent on the consumer’s desires.

